Global Licensing
In today’s increasingly interconnected and globalised world, strategic alliances are key drivers for growth and innovation.We facilitate in-licensing and out-licensing partnerships that bring together companies with complementary competencies to strengthen pipelines, achieve economies of scale, advance technologies, and introduce commercial products to market.
With a robust biologics portfolio of finished products as well as bulk from our strategic partners, we make a staged backward integration project viable. Many of our clients have secured long-term supply contracts with the government in countries where local manufacturing is supported by the authorities. We also support international specialty pharmaceutical companies looking to advance their assets in newer markets through partnerships with local pharmaceutical companies and distributors, providing support for regulatory approval and commercialization of the products in each market.

A typical staged backward integration model starts with finished product registration and supplies but often transitions into secondary packaging, followed by local filling and QC release, and eventually formulation filling and QC release. A further backward integration step of API/DS manufacturing is also possible in some cases.
Technology Transfer
For each stage from lab scale to pilot to commercial scales, our team ensures process optimization for higher yields while also focusing on batch-to-batch consistency based output, whether the tech transfer is within the organization or outsourced or out-licensed at any stage. We also assist you with licensing by conducting thorough due diligence, allowing you to close the deal with no stress.

Our deliverables include
- End-to-end tech transfers for polysaccharide, recombinant and toxoid based vaccine development and commercial manufacturing
- End-to-end tech transfer support for biosimilar development and manufacturing
- Supplies of finished products, unformulated, formulated (ready-to-fill bulks) for various vaccine antigens and biosimilar products
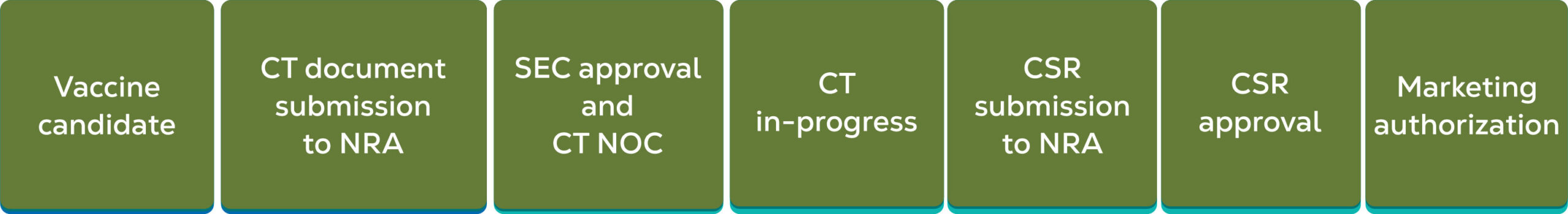
Clinical Trials
TechInvention is committed to providing the best clinical trial management services for biologicals. These services allow healthcare companies to confidently try new things, make the most of opportunities, and improve global health.
Because biologicals are more complex than chemical drugs, both in composition and method of manufacture, the clinical trial process is equally complex. At TechInvention, we understand the challenges involved and have established all the necessary infrastructure needed to generate robust, clinically validated data for biologicals. We have invested our resources in clinically assessing biologicals against disease strains prevalent in the developing world, which bears 90% of the global burden of disease.

We also form clinical trial partnerships with governments, policymakers, multilateral and international organizations, the public-private sector, research and technical institutes, non-governmental organizations (NGOs), philanthropic organizations, and other like-minded organizations with the goal of long-term strengthening of public health systems and immunization programs in developing countries.
Our deliverables include
- Liaise with CDSCO and DCGI to initiate trials.
- Oversee the quality and integrity of trial data outsourced to CROs, ensuring the data is reliable and has been processed correctly.
- Secure agreements from all parties to ensure direct access to trial-related sites, source data and documents, and reports for monitoring and auditing by the sponsor, as well as inspection by domestic and foreign regulatory authorities.
- Ensure compliance with the protocol, GCP, and applicable regulatory requirements.
- Appoint qualified individuals to supervise the overall conduct of the trial, verify the data, conduct the statistical analyses, and to prepare the trial reports.
- Participate in investigator selection, compensation to subjects and investigators, financing, ADR reporting, monitoring and auditing.
In-licenced Product
Cholera
- Package Insert (Download PDF)
- Summary of Product Characterization (Download PDF)