-
We are now ISO 13485:2016 certified!
The ISO 13485:2016 standard is globally recognized for quality management systems specific to the medical device industry. Achieving this milestone demonstrates that our HORIZON facility meets stringent international requirements for the design, development, production, and distribution of medical devices.

-
We're Forbes Recognized!
Honoured to be named in Forbes India’s SELECT 200 at DGEMS 2024 and to engage with Forbes India and The D Globalist. This recognition strengthens our commitment to global innovation and impact.

-
Our partnership
Our partnership with Quantoom Biosciences marks a significant milestone in expanding India’s GMP RNA manufacturing capabilities

-
Foundations of Bioprocessing: A Hands-On Introductory Workshop
We collaborated with BSc BioNEST Bio-incubator at the Regional Centre for Biotechnology to deliver an impactful hands-on training session aimed at nurturing India’s next generation of scientific talent.

-
ORBIT: ‘One Health Research & Bio-Innovation Technology Hub’ launched at Ganpat University by TechInvention
Established at Ganpat University with CSR support and TechInvention’s technical partnership, ORBIT is a Centre of Excellence driving translational research on antibodies, diagnostics, and vaccines for human and animal health.

-
Foundations of Bioprocessing: A Hands-On Introductory Workshop
We collaborated with BSc BioNEST Bio-incubator at the Regional Centre for Biotechnology to deliver an impactful hands-on training session aimed at nurturing India’s next generation of scientific talent.

-
New Publication!
TechInvention proposes actionable strategies to empower LMICs with affordable, accessible, and equitable medical countermeasures for future health emergencies.

-
New Publication!
Our review highlights the shifting serotype landscape of pneumococcal disease in South Asia and advocates for region-specific PCV strategies and alternative vaccine approaches to address emerging challenges.

-
We are now ISO 13485:2016 certified!
The ISO 13485:2016 standard is globally recognized for quality management systems specific to the medical device industry. Achieving this milestone demonstrates that our HORIZON facility meets stringent international requirements for the design, development, production, and distribution of medical devices.

-
We're Forbes Recognized!
Honoured to be named in Forbes India’s SELECT 200 at DGEMS 2024 and to engage with Forbes India and The D Globalist. This recognition strengthens our commitment to global innovation and impact.

-
Our partnership
Our partnership with Quantoom Biosciences marks a significant milestone in expanding India’s GMP RNA manufacturing capabilities

-
Foundations of Bioprocessing: A Hands-On Introductory Workshop
We collaborated with BSc BioNEST Bio-incubator at the Regional Centre for Biotechnology to deliver an impactful hands-on training session aimed at nurturing India’s next generation of scientific talent.

-
ORBIT: ‘One Health Research & Bio-Innovation Technology Hub’ launched at Ganpat University by TechInvention
Established at Ganpat University with CSR support and TechInvention’s technical partnership, ORBIT is a Centre of Excellence driving translational research on antibodies, diagnostics, and vaccines for human and animal health.

-
Foundations of Bioprocessing: A Hands-On Introductory Workshop
We collaborated with BSc BioNEST Bio-incubator at the Regional Centre for Biotechnology to deliver an impactful hands-on training session aimed at nurturing India’s next generation of scientific talent.

-
New Publication!
TechInvention proposes actionable strategies to empower LMICs with affordable, accessible, and equitable medical countermeasures for future health emergencies.

-
New Publication!
Our review highlights the shifting serotype landscape of pneumococcal disease in South Asia and advocates for region-specific PCV strategies and alternative vaccine approaches to address emerging challenges.

Enabling One Health through Bio-Innovation

Research & Development

Strategic Advisory & Tech Consulting

In & Out Licensing

Contract Development & Manufacturing
HORIZON
High-impact One-health Research & Innovation ZONe

A GMP-like facility thoughtfully designed with innovative and sustainable concepts, aimed at enabling efficient and environmentally responsible production of preclinical vaccine batches.
GCMC
Global Collaborative Centre for Medical Countermeasures

An EU-GMP-compliant facility purpose-built to enable seamless scale-up and accelerate the translation of vaccine candidates from the pre-clinical stage through clinical development to full-scale commercialization.
This continuum enables TechInvention to support LMIC-focused innovation with speed, efficiency, and quality.
Antimicrobial resistance (AMR) continues to severely affect global health and the global economy. It is one of the most serious public health threats of the 21st century, exacerbated by antibiotic overuse and misuse worldwide.It is responsible for 700,000 deaths annually and is predicted to cause more than 10 million deaths by 2050. It is essential to take the One Health approach to effectively address the AMR issue. The concept of One Health refers to an all-encompassing strategy for improving the well-being of people, animals, and the environment.
To enable and support One Health, TechInvention employs four strategies: research and development, contract development and manufacturing for scale-ups and commercial production, technical and strategic advisory for capacity building, and global access to diagnostics, vaccines, and biopharmaceuticals.

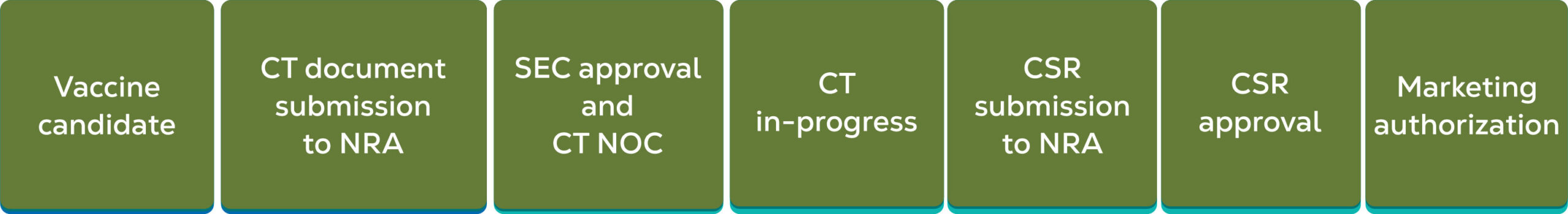
Regulatory Status of In-licensed Products
Global Project & Partners




